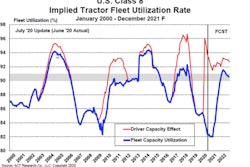
Whether it’s next week or next year, trucking will play no small role in deploying COVID-19 vaccines around the U.S. The hauls are likely to be trickier and more sensitive than other types of freight, and the extreme temperature requirements of some of the vaccines poses hurdles. With capacity already tight, an infusion of vaccine freight could cause delays for other time-sensitive, refrigerated goods. Nonetheless, from truckload to final mile, carriers and logistics personnel say they’re ready to go when the vaccine is.
A wave of good news over the past week from major pharmaceutical companies regarding the success of experimental COVID-19 vaccines could lead to the deployment of tens of millions of doses to be administered by year’s end.
Pfizer, who said its vaccine trial showed an effective rate of 95%, hopes to administer some 50 million vaccine doses by the end of next month. Likewise, Moderna, who on Monday announced a 94.5% effective rate for its COVID vaccine, said it hopes to distribute 20 million doses before New Year’s Day.
Use of those vaccines is still dependent upon FDA stamping approval on the companies’ requests for emergency use. Nonetheless, getting millions of vaccine doses – some of which are required to be kept at -70 degrees Celsius (almost 100 degrees below 0 degrees Fahrenheit) – to the hospitals, pharmacies and clinics where they’ll be administered will take a coordinated supply chain effort and more than a few very cold reefer trailers.
The temperature range of the Moderna vaccine falls well within the typical range of the refrigerated pharmaceutical loads handled by Bio Pharma Logistics (BPL), said Rick Hartlage, BPL’s chief operating officer. BPL is an asset-based carrier that specializes in hauling frozen blood plasma as a raw material for pharma companies to use in making medicines and, currently, treatments for COVID-19. Hartlage said BPL’s loads typically are transported at between -29 Celsius and -35 Celsius.
Unlike Moderna’s vaccine, the extreme cold temperature requirements of Pfizer’s vaccine poses “hurdles that are going to have to be overcome,” said Hartlage.